| [1] |
A. Aitken, Trends Cell Biol. 6 (1996) 341-347. |
| [2] |
K. Altendorf, A. Siebers, W. Epstein, Ann. N.Y. Acad. Sci. 671 (1992) 228-243. |
| [3] |
I.T. Arkin, P.D. Adams, A.T. Brunger, S.O. Smith, D.M. Engelman, Ann. Rev. Biophys. Biomol. Struct. 26 (1997) 157-179. |
| [4] |
K.B. Axelsen, M.G. Palmgren, J. Mol. Evol. 46 (1998) 84-101. |
| [5] |
L. Baunsgaard, A.T. Fuglsang, T. Jahn, H.A.A.J. Korthout, A.H. de Boer, M.G. Palmgren, Plant J. 13 (1998) 661-667. |
| [6] |
P. Beguin, M.C. Peitsch, K. Geering, Biochemistry 35 (1996) 14098-14108. |
| [7] |
P. Beguin, X.Y. Wang, D. Firsov, A. Puoti, D. Claeys, J.D. Horisberger, K. Geering, EMBO J. 16 (1997) 4250-4260. |
| [8] |
F.R. Blattner et al., Science 277 (1997) 1453-1474. |
| [9] |
C.J. Bult et al., Science 273 (1996) 1058-1073. |
| [10] |
E.T. Buurman, K.T. Kim, W. Epstein, J. Biol. Chem. 270 (1995) 6678-6685. |
| [11] |
P. Catty, A.D. D'Exaerde, A. Goffeau, FEBS Lett. 409 (1997) 325-332. |
| [12] |
N. Courtois-Coutry, D. Roush, V. Rajendran, J.B. McCarthy, J. Geibel, M. Kashgarian, M.J. Caplan, Cell 90 (1997) 501-510. |
| [13] |
K.W. Cunningham, G.R. Fink, J. Cell Biol. 124 (1994) 351-363. |
| [14] |
L.A. Dunbar, D.L. Roush, N. Courtois-Coutry, T.R. Muth, C.J. Gottardi, V. Rajendran, J. Geibel, M. Kashgarian, M.J. Caplan, Ann. N.Y. Acad. Sci. 834 (1997) 514-523. |
| [15] |
A. Enyedi, T. Vorherr, P. James, D.J. McCormick, A.G. Filoteo, E. Carafoli, J.T. Penniston, J. Biol. Chem. 264 (1989) 12313-12321. |
| [16] |
P. Eraso, F. Portillo, J. Biol. Chem. 269 (1994) 10393-10399. |
| [17] |
R. Falchetto, T. Vorherr, J. Brunner, E. Carafoli, J. Biol. Chem. 266 (1991) 2930-2936. |
| [18] |
M.J. Fagan, M.H. Saier Jr., J. Mol. Evol. 38 (1994) 57-99. |
| [19] |
R. Falchetto, T. Vorherr, E. Carafoli, Protein Sci. 1 (1992) 1613-1621. |
| [20] |
N. Ferrol, A.B. Bennett, Plant Cell 8 (1996) 1159-1169. |
| [21] |
M.S. Feschenko, K.J. Sweadner, J. Biol. Chem. 272 (1997) 17726-17733. |
| [22] |
M.S. Feschenko, R.K. Wetzel, K.J. Sweadner, Ann. N.Y. Acad. Sci. 834 (1997) 479-488. |
| [23] |
G. Fisone et al., J. Biol. Chem. 269 (1994) 9368-9373. |
| [24] |
R.D. Fleischmann et al., Science 269 (1995) 496-512. |
| [25] |
C.M. Fraser et al., Science 270 (1995) 397-403. |
| [26] |
C.M. Fraser et al., Nature 390 (1995) 580-586. |
| [27] |
K. Geering, A. Beggah, P. Good, S. Girardet, S. Roy, D. Schaer, P. Jaunin, J. Cell Biol. 133 (1996) 1193-1204. |
| [28] |
A. Goffeau et al., Nature 387(Suppl.) (1997) 5-105. |
| [29] |
R.M. Gopinath, F.F. Vincenci, Biochem. Biophys. Res. Commun. 77 (1977) 1203-1209. |
| [30] |
J.F. Harper, B. Hong, I. Hwang, H.Q. Guo, R. Stoddard, J.F. Huang, M.G. Palmgren, H. Sze, J. Biol. Chem. 273 (1998) 1099-1106. |
| [31] |
Hebsgaard, S.M., Korning, P.G., Tolstrup, N., Engelbrecht, J., Rouze, P. and Brunak, S. (1996) Nucleic Acids Res. 24, 3439-3452. |
| [32] |
R. Himmelreich, H. Hilbert, H. Plagens, E. Pirkl, B.C. Li, R. Herrmann, Nucleic Acids Res. 24 (1996) 4420-4449. |
| [33] |
L. Huang, T. Berkelman, A.E. Franklin, N.E. Hoffman, Proc. Natl. Acad. Sci. USA 90 (1993) 10066-10070. |
| [34] |
T. Jahn, A.T. Fuglsang, A. Olsson, I.M. Brüntrup, D.B. Collinge, D. Volkmann, M. Sommarin, M.G. Palmgren, C. Larsson, Plant Cell 9 (1997) 1805-1814. |
| [35] |
P. James, M. Maeda, R. Fischer, A.K. Verma, J. Krebs, J.T. Penniston, E. Carafoli, J. Biol. Chem. 263 (1988) 2905-2910. |
| [36] |
H.W. Jarrett, J.T. Penniston, Biochem. Biophys. Res. Commun. 77 (1977) 1210-1216. |
| [37] |
P.L. Jørgensen, J.P. Andersen, J. Membr. Biol. 103 (1988) 95-120. |
| [38] |
P.L. Jørgensen, J.H. Rasmussen, J.M. Nielsen, P.A. Pedersen, Ann. N.Y. Acad. Sci. 834 (1997) 161-174. |
| [39] |
T. Kaneko et al., DNA Res. 3 (1996) 109-136. |
| [40] |
H.-P. Klenk et al., Nature 390 (1997) 364-370. |
| [41] |
F. Kunst et al., Nature 390 (1997) 249-256. |
| [42] |
J.B. Lingrel, J.M. Argüello, J. van Huysse, T.A. Kuntzweiler, Ann. N.Y. Acad. Sci. 834 (1997) 194-206. |
| [43] |
N.S. Logvinenko, I. Dulubova, N. Fedosova, S.H. Larsson, A.C. Nairn, M. Esmann, P. Greengard, A. Aperia, Proc. Natl. Acad. Sci. U.S.A. 93 (1996) 9132-9137. |
| [44] |
S. Lutsenko, J.H. Kaplan, Biochemistry 34 (1995) 15607-15612. |
| [45] |
S. Lutsenko, K. Petrukhin, T.C. Gilliam, J.H. Kaplan, Ann. N.Y. Acad. Sci. 834 (1997) 155-157. |
| [46] |
D.H. MacLennan, W.J. Rice, A. Odermatt, Ann. N.Y. Acad. Sci. 834 (1997) 175-185. |
| [47] |
S. Malmström, P. Askerlund, M.G. Palmgren, FEBS Lett. 400 (1997) 324-328. |
| [48] |
J. Moniakis, M.B. Coukell, A. Forer, J. Biol. Chem. 270 (1995) 28276-28281. |
| [49] |
P. Morsomme, A. de Kerchove d'Exaerde, S. De Meester, D. Thinès, A. Goffeau, M. Boutry, EMBO J. 15 (1996) 5513-526. |
| [50] |
A.J. Muslin, J.W. Tanner, P.M. Allen, A.S. Shaw, Cell 84 (1996) 889-897. |
| [51] |
J.V. Møller, B. Juul, M. le Maire, Biochim. Biophys. Acta 1286 (1996) 1-51. |
| [52] |
C. Navarre, P. Catty, S. Leterme, F. Dietrich, A. Goffeau, J. Biol. Chem. 269 (1994) 21262-21268. |
| [53] |
C. Oecking, M. Piotrowski, J. Hagemeier, K. Hagemann, Plant J. 12 (1997) 441-453. |
| [54] |
M.G. Palmgren, Adv. Bot. Res. 28 (1998) 1-70. |
| [55] |
M.G. Palmgren, M. Sommarin, R. Serrano, C. Larsson, J. Biol. Chem. 266 (1991) 20470-20475. |
| [56] |
F. Portillo, I.F. de Larrinoa, R Serrano, FEBS Lett. 247 (1989) 381-385. |
| [57] |
F. Portillo, P. Eraso, R. Serrano, FEBS Lett. 287 (1991) 71-74. |
| [58] |
B. Regenberg, J.M. Villalba, F.C. Lanfermeier, M.G. Palmgren, Plant Cell 7 (1995) 1655-1666. |
| [59] |
R. Serrano, FEBS Lett. 156 (1983) 11-14. |
| [60] |
D.R. Smith et al., J. Bacteriol. 179 (1997) 7135-7155. |
| [61] |
A. Sorin, G. Rosas, R. Rao, J. Biol. Chem. 272 (1997) 9895-9901. |
| [62] |
K.T. Togawa, T. Ishiguro, K. Kaya, A. Shimada, T. Imagawa, K. Taniguchi, J. Biol. Chem. 270 (1995) 15475-15478. |
| [63] |
J.F. Tomb et al., Nature 388 (1997) 539-547. |
| [64] |
A.K. Verma, A. Enyedi, A.G. Filoteo, J.T. Penniston, J. Biol. Chem. 269 (1994) 1687-1691. |
| [65] |
B. Vilsen, D. Ramlov, J.P. Andersen, Ann. N.Y. Acad. Sci. 834 (1997) 297-309. |
| [66] |
T. Vorherr, M. Chiesi, R. Schwaller, E. Carafoli, Biochemistry 31 (1992) 371-376. |
| [67] |
M.B. Yaffe, K. Rittinger, S. Volinia, P.R. Caron, A. Aitken, H. Leffers, S.J. Gamblin, S.J. Smerdon, L.C. Cantley, Cell 91 (1997) 961-971. |
| [68]
| M. Zurini, J. Krebs, J.T. Penniston, E. Carafoli, J. Biol. Chem. 259 (1984) 618-627. |
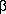 subunit in addition to the catalytic
subunit in addition to the catalytic  subunit. The
subunit. The 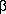 subunit seems to be important for maturation during transport through the secretory pathway [27], for intracellular sorting in polarized epithelial cells [12] and for determining ion binding properties of the
subunit seems to be important for maturation during transport through the secretory pathway [27], for intracellular sorting in polarized epithelial cells [12] and for determining ion binding properties of the  subunit. The evolutionary origin of the
subunit. The evolutionary origin of the 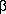 subunit is not known, but it has been suggested that it represents a remnant of the bacterial KdpC subunit [4]. In addition to the
subunit is not known, but it has been suggested that it represents a remnant of the bacterial KdpC subunit [4]. In addition to the 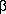 subunit, the Na+/K+-ATPase may be associated with a small proteolipid, the
subunit, the Na+/K+-ATPase may be associated with a small proteolipid, the 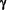 subunit, which seems to be important for modulating K+ activation of the pump [7]. The plasma membrane H+-ATPase (type IIIA) of S. cerevisiae is likewise associated with a small proteolipid for which two genes exist, PMP1 and PMP2 [52]. The function of this component remains obscure.
subunit, which seems to be important for modulating K+ activation of the pump [7]. The plasma membrane H+-ATPase (type IIIA) of S. cerevisiae is likewise associated with a small proteolipid for which two genes exist, PMP1 and PMP2 [52]. The function of this component remains obscure.