P-type ATPase references
The following list of references is by no means exhaustive, as there are (far) more than 5000 articles about P-type ATPases. It is meant to provide an entrance to the P-type ATPase field and comments and suggestions to improve it are very welcome.
This first article was the first linking ion transport to ATPase activity, a discovery that led to the Nobel Prize in Chemistry to J. C. Skou in 1997.
- Skou, J. C. (1957) The Influence of Some Cations on an Adenosine Triphosphatase from Peripheral Nerves. Biochim. Biophys. Acta 23: 394-401.
The article can also be found in the millenium volume of Biochimica et Biophysica Acta together with a comment on the original work written by J. C. Skou himself.
- Skou, J. C. (1989) The Influence of Some Cations on an Adenosine Triphosphatase from Peripheral Nerves. 1957 [classical article]. Biochim. Biophys. Acta 1000: 439-446.
- Skou, J. C. (1989) The identification of the sodium-pump as the membrane-bound Na+/K+- ATPase: a commentary on 'The Influence of Some Cations on an Adenosine Triphosphatase from Peripheral Nerves'. Biochim. Biophys. Acta 1000: 435-438.
The next articles provides the basis for this database. Articles providing structural information about P-type ATPases.
- Toyoshima, C., and Mizutani, T. (2004) Crystal structure of the calcium pump with a bound ATP analogue. Nature 430: 529-535.
- Håkansson, K.O. (2003) The crystallographic structure of Na,K-ATPase N-domain at 2.6Å resolution. J. Mol. Biol. 332: 1175-1182.
- Hilge, M., Siegal, G., Vuister, G.W., Güntert, P., Gloor, S.M., and Abrahams, J.P. (2003) ATP-induced conformational changes of the nucleotide-binding domain of Na,K-ATPase. Nat. Struct. Biol. 10: 468-474.
- Toyoshima, C., and Nomura, H. (2002) Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418: 605-611.
- Kühlbrandt, W., Zeelen, J., and Dietrich, J. (2002) Structure, Mechanism, and Regulation of the Neurospora Plasma Membrane H+-ATPase. Science 297: 1692-1696.
- Xu, C., Rice, W.J., He, W., and Stokes, D.L. (2002) A structural model for the catalytic cycle of Ca2+-ATPase. J. Mol. Biol. 316: 201-211.
- Toyoshima, C., Asahi, M., Sugita, Y., Khanna, R., Tsuda, T., and MacLennan, D.H. (2003) Modeling of the inhibitory interaction of phospholamban with the Ca2+ ATPase. Proc. Natl. Acad. Sci. U.S.A. 100: 467-472.
- Ogawa, H. and Toyoshima, C. (2002) Homology modeling of the cation binding sites of Na+K+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 99: 15977-15982.
- Toyoshima, C., Nakasako, M., Nomura, H., and Ogawa, H. (2000) Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405: 647-655.
- Auer, M., Scarborough, G. A., and Kühlbrandt, W. (1998) Three-dimensional map of the plasma membrane H+-ATPase in the open conformation. Nature 392: 840-843.
- Zhang, P., Toyoshima, C., Yonekura, K., Green, N. M., and Stokes, D. L. (1998) Structure of the calcium pump from sarcoplasmic reticulum at 8-Å resolution. Nature 392: 835-839.
- Kühlbrandt, W., Auer, M., and Scarborough, G. A. (1998) Structure of the P-type ATPases. Curr. Opin. Struct. Biol. 8: 510-516.
- Gitschier, J., Moffat, B., Reilly, D., Wood, W. I., and Fairbrother, W. J. (1998) Solution structure of the fourth metal-binding domain from the Menkes copper-transporting ATPase. Nat. Struct. Biol. 5: 47-54.
- Elshorst, B., Hennig, M., Försterling, H., Diener, A., Maurer, M., Schulte, P., Schwalbe, H., Griesinger, C., Krebs, J., Schmid, H., Vorherr, T., and Carafoli, E. (1999) NMR solution structure of a complex of calmodulin with a binding peptide of the Ca2+ pump. Biochemistry 38:12320-12332.
- Aravind, L., Galperin, M. Y., and Koonin, E. V. (1998) The catalytic domain of the P-type ATPase has the haloacid dehalogenase fold. Trends Biochem. Sci. 23: 127-129.
- Ridder, I. S. and Dijkstra, B. W. (1999) Identification of the Mg2+-binding site in the P-type ATPase and phosphatase members of the HAD (haloacid dehalogenase) superfamily by structural similarity to the response regulator protein CheY. Biochem. J. 339: 223-226.
- Stokes, D. L., Taylor, W. R., and Green, N. M. (1994) Structure, transmembrane topology and helix packing of P-type ion pumps. FEBS Lett. 346: 32-38.
- Champeil, P., Menguy, T., Soulié, S., Juul, B., de Gracia, A. G., Rusconi, F., Falson, P., Denoroy, L., Henao, F., Le Maire, M., and Møller, J. V. (1998) Characterization of a protease-resistant domain of the cytosolic portion of sarcoplasmic reticulum Ca2+-ATPase. Nucleotide- and metal- binding sites. J. Biol. Chem. 273: 6619-6631.
Reviews about P-type ATPases. Pedersen and Carafoli suggested the name P-type ATPases to replace E1-E2
ATPases.
- Pedersen, P. L., and Carafoli, E. (1987) Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem. Sci. 4: 146-150.
- Jørgensen, P. L., Nielsen, J. M., Rasmussen, J. H., and Pedersen, P. A. (1998) Structure-function relationships of E1-E2 transitions and cation binding in Na, K-pump protein. Biochim. Biophys. Acta 1365: 65-70.
- MacLennan, D. H., Rice, W. J., and Green, N. M. (1997) The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+- ATPases. J. Biol. Chem. 272: 28815-28818.
- Møller, J. V., Juul, B., and Le Maire, M. (1996) Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim. Biophys. Acta 1286: 1-51.
- Solioz, M., and Vulpe, C. (1996) CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem. Sci. 21: 237-241.
- Silver, S., and Phung, L. T. (1996) Bacterial heavy metal resistance: new surprises. Annu. Rev. Microbiol. 50: 753-789.
- Rice, W. J., and MacLennan, D. H. (1996) Scanning mutagenesis reveals a similar pattern of mutation sensitivity in transmembrane sequences M4, M5, and M6, but not in M8, of the Ca2+- ATPase of sarcoplasmic reticulum (SERCA1a). J. Biol. Chem. 271: 31412-31419.
- Lutsenko, S., and Kaplan, J. H. (1995) Organization of P-type ATPase: Significance of structural diversity. Biochemistry 34: 15607-15612.
- Lewis, K. (1994) Multidrug resistance pumps in bacteria: variations on a theme. Trends Biochem. Sci. 19: 119-123.
The newest substrates shown to be transported by P-type ATPases: zinc, lead, cobalt, and maybe phospholipids.
- Thelwell, C., Robinson, N. J., and Turner-Cavet, J. S. (1998) An SmtB-like repressor from Synechocystis PCC 6803 regulates a zinc exporter. Proc. Natl. Acad. Sci. U. S. A. 95: 10728-10733.
- Beard, S. J., Hashim, R., Membrillo-Hernandez, J., Hughes, M. N., and Poole, R. K. (1997) Zinc(II) tolerance in Escherichia coli K-12: evidence that the zntA gene (o732) encodes a cation transport ATPase. Mol. Microbiol. 25: 883-891.
- Rensing, C., Mitra, B., and Rosen, B. P. (1997) The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. U. S. A. 94: 14326-14331.
- Rensing, C., Sun, Y., Mitra, B., and Rosen, B. P. (1998) Pb(II)-translocating P-type ATPases. J. Biol. Chem. 273: 32614-32617. (E. coli ZntA or S. aureus CadA)
- Rutherford, J.C., Cavet, J.S., and Robinson, N.J. (1999) Cobalt-dependent transcriptional switching by a dual-effector MerR-like protein regulates a cobalt-exporting variant CPx-type ATPase. J. Biol. Chem. 274: 25827-25832. (Synechocystis PCC6803 CoaT)
- Auland, M. E., Roufogalis, B. D., Devaux, P. F., and Zachowski, A. (1994) Reconstitution of ATP-dependent aminophospholipid translocation in proteoliposomes. Proc. Natl. Acad. Sci. U. S. A. 91: 10938-10942.
- Tang, X., Halleck, M. S., Schlegel, R. A., and Williamson, P. (1996) A subfamily of P-type ATPases with aminophospholipid transporting activity. Science 272: 1495-1497.
These articles showed the existance of two conformational steps during the P-type ATPase catalytic cycle.
- Jørgensen, P. L. (1975) Purification and characterization of (Na+, K+)-ATPase. V. Conformational changes in the enzyme transitions between the Na-form and the K-form studied with tryptic digestion as a tool. Biochim. Biophys. Acta 401: 399-415.
- Andersen, J. P., and Jørgensen, P. L (1985) Conformational states of sarcoplasmic reticulum Ca2+-ATPase as studied by proteolytic cleavage. J. Membr. Biol. 88: 187-198.
Defective P-type ATPases in humans are the cause of severe diseases, such as Menkes syndrome (ATP7A), Wilson disease (ATP7B), Brody myopathy (ATP2A1), Darier-White disease (ATP2A2), benign chronic pemphigus also known as Hailey-Hailey disease (ATP2C1), hereditary cholestasis also known as Bylers disease (ATP8B1), and Angelman syndrome (ATP10A). Misrouting of the FXYD subunit of the P2C ATPases leads to renal hypomagnesemia.
- Vulpe, C., Levinson, B., Whitney, S., Packman, S., and Gitschier, J. (1993) Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 3: 7-13.
- Chelly, J., Tümer, Z., Tønnesen, T., Petterson, A., Ishikawa-Brush, Y., Tommerup, N., Horn, N., and Monaco, A.P. (1993) Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat. Genet. 3: 14-19.
- Mercer, J.F., Livingston, J., Hall, B., Paynter, J.A., Begy, C., Chandrasekharappa, S., Lockhart, P., Grimes, A., Bhave, M., Siemieniak, D., and Glower, T.W. (1993) Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat. Genet. 3: 20-25.
- Bull, P.C., Thomas, G.R., Rommens, J.M., Forbes, J.R., and Cox, D.W. (1993) The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 5: 327-337.
- Petrukhin, K., Fischer, S.G., Pirastu, M., Tanzi, R.E., Chernov, I., Devoto, M., Brzustowicz, L.M., Cayanis, E., Vitale, E., and Russo, J.J., Matseoane, D., Boukhgalter, B., Wasco, W., Figus, A.L., Loudianos, J., Cao, A., Sternlieb, I., Evgrafov, O., Parano, E., Pavone, L., Warburton, D., Ott, J., Penchaszadeh, G.K., Scheinberg, I.H., and Gilliam, T.C. (1993) Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat. Genet. 5: 338-343.
- Tanzi, R.E., Petrukhin, K., Chernov, I., Pellequer, J.L., Wasco, W., Ross, B., Romano, D.M., Parano, E., Pavone, L., Brzustowicz, L.M. Devoto, M., Peppercorn, J., Bush, A.I., Sternlieb, I., Pirastu, M., Gusella, J.F., Evgrafov, O., Penchaszadeh, G.K., Honig, B., Edelman, I.S., Soares, M.B., Scheinberg, I.H., and Gilliam, T.C. (1993) The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 5: 344-350.
- Odermatt, A., Taschner, P.E., Khanna, V.K., Busch, H.F., Karpati, G., Jablecki, C.K., Breuning, M.H., and MacLennan, D.H. (1996) Mutations in the gene-encoding SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with Brody disease. Nat. Genet. 14: 191-194.
- Sakuntabhai, A., Ruiz-Perez, V., Carter, S., Jacobsen, N., Burge, S., Monk, S., Smith, M., Munro, C.S., O'Donovan, M., Craddock, N., Kucherlapati, R., Rees, J.L., Owen, M., Lathrop, G.M., Monaco, A.P., Strachan, T., and Hovnanian, A. (1999) Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat. Genet. 21: 271-277.
- Hu Z., Bonifas J.M., Beech J., Bench G., Shigihara T., Ogawa H., Ikeda S., Mauro T., Epstein E.H. Jr. (2000) Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat. Genet. 24: 61-65.
- Bull, L.N., van Eijk, M.J.T., Pawlikowska, L., DeYoung, J.A., Juijn, J.A., Liao, M., Klomp, L.W.J., Lomri, N., Berger, R., Scharschmidt, B.F., Knisely, A.S., Houwen, R.H.J., and Freimer, N.B. (1998) A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat. Genet. 18: 219-224.
- Meguro, M., Kashiwagi, A., Mitsuya, K., Nakao, M., Kondo, I., Saitoh, S., and Oshimura M. (2001) A novel maternally expressed gene, ATP10C, encodes a putative aminophospholipid translocase associated with Angelman syndrome. Nat. Genet. 28:19-20.
- Meij, I.C., Koenderink, J.B., van Bokhoven, H., Assink, K.F.H., Groenestege, W.T., de Pont, J.J.H.H.M., Bindels, R.J.M., Monnens, L.A.H., van den Heuvel, L.P.W.J., Knoers, N.V.A.M. (2000) Dominant isolated renal magnesium loss is caused by misrouting of the Na+,K+-ATPase
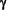 -subunit. Nat. Genet. 26: 265-266.
-subunit. Nat. Genet. 26: 265-266.
An article providing information on the possible soluble ancestor of P-type ATPases.
Go to Front page
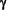 -subunit. Nat. Genet. 26: 265-266.
-subunit. Nat. Genet. 26: 265-266.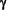 -subunit. Nat. Genet. 26: 265-266.
-subunit. Nat. Genet. 26: 265-266.